Background
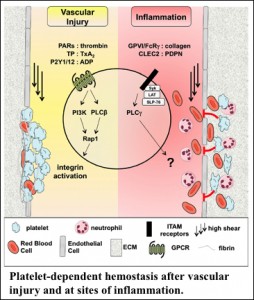
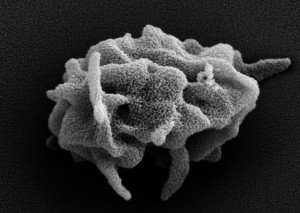
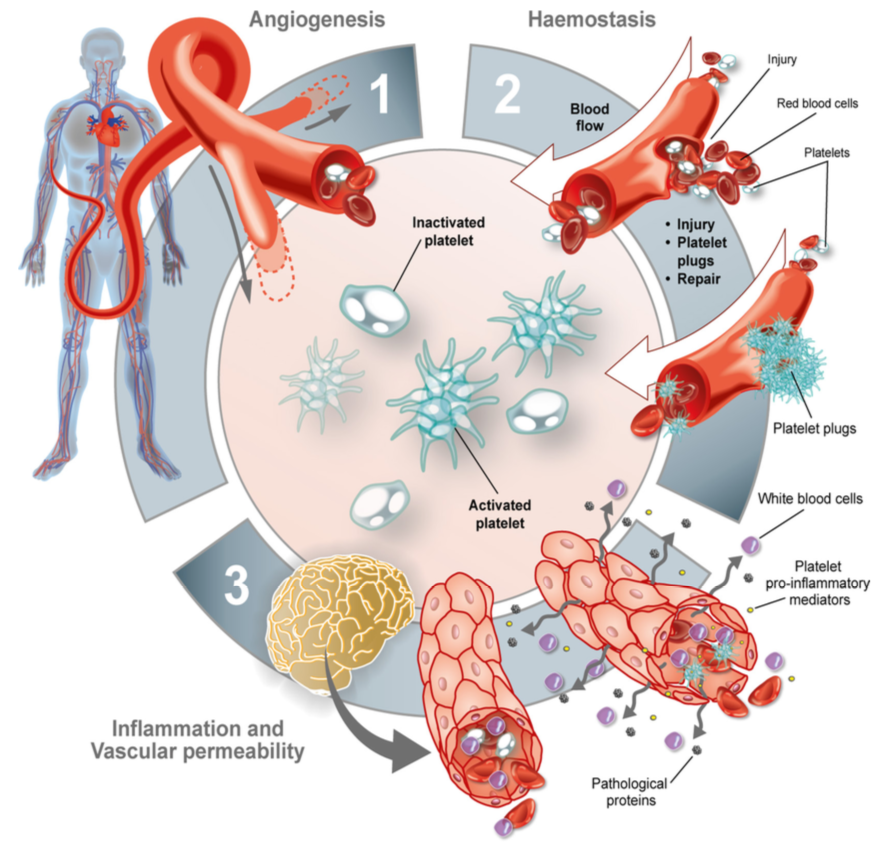
Blood platelets are critical for diverse physiological processes such as hemostasis, angiogenesis, and innate immunity. Every day ~100 billion platelets are produced by megakaryocytes (MKs) in the bone marrow and destroyed by phagocytic cells of the reticulo-endothelial system. Defects in either process can lead to thrombocytopenia (low platelet count) or thrombocytosis (high platelet count). While thrombocytosis is a risk factor for various clinical complications, including venous thrombosis and cancer, thrombocytopenia is a common cause of bleeding complications. Hemostatic plug formation under shear stress conditions at sites of vascular injury depends on the platelets’ ability to sense and to rapidly respond to disruptions in the vascular wall, processes that strongly depend on G protein signaling. At the same time, strong negative regulators ensure that platelets remain in a quiescent state while in circulation and that thrombus formation is a self-limiting process.
Bergmeier Lab Achievements
My lab has made major contributions to our understanding of platelet G protein signaling and the mechanisms regulating platelet reactivity and platelet count.

Significant findings include:
• Rap and Rho small GTPases as critical regulators of megakaryocytic and platelet function.
• Rap1 signaling as an important rheostat for platelet adhesive function.
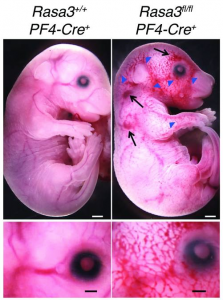
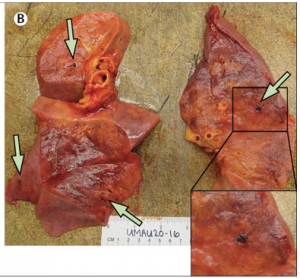
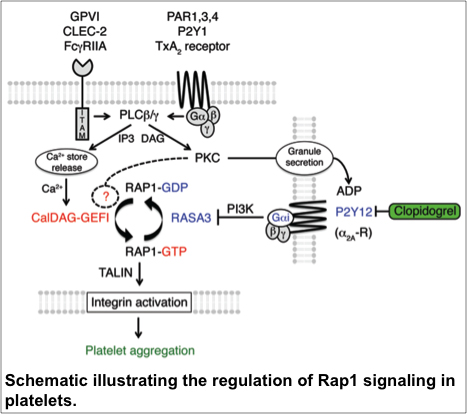
• Injury-specific contributions of platelets to vascular integrity. Mutations in the Rap-GEF, CalDAG-GEFI, lead to impaired platelet function and bleeding in humans.
• Improved understanding of how currently used antiplatelet drugs work and why platelet transfusion therapy often fails.
• Novel mouse models to investigate small GTPase signaling in MKs and platelets.
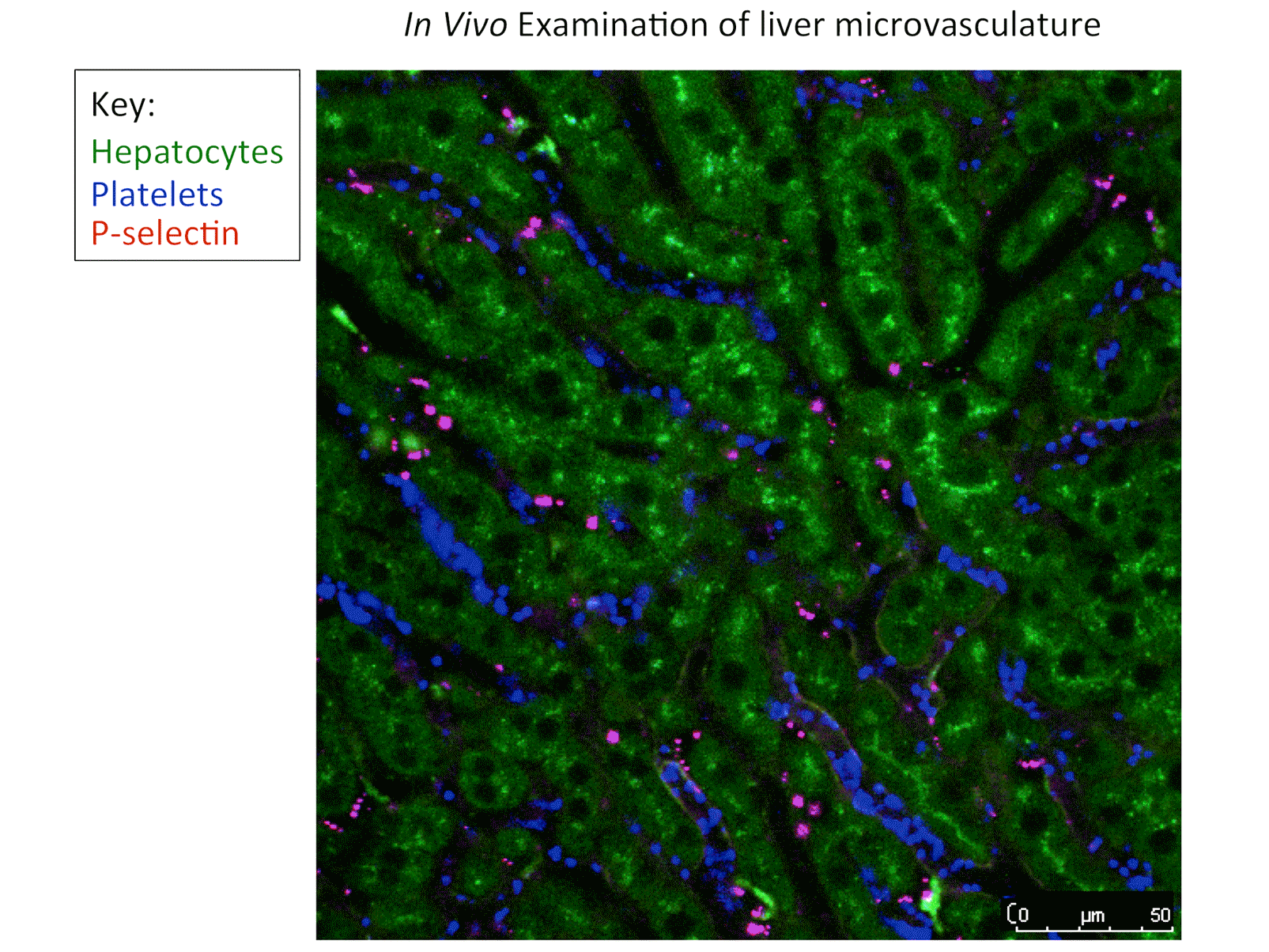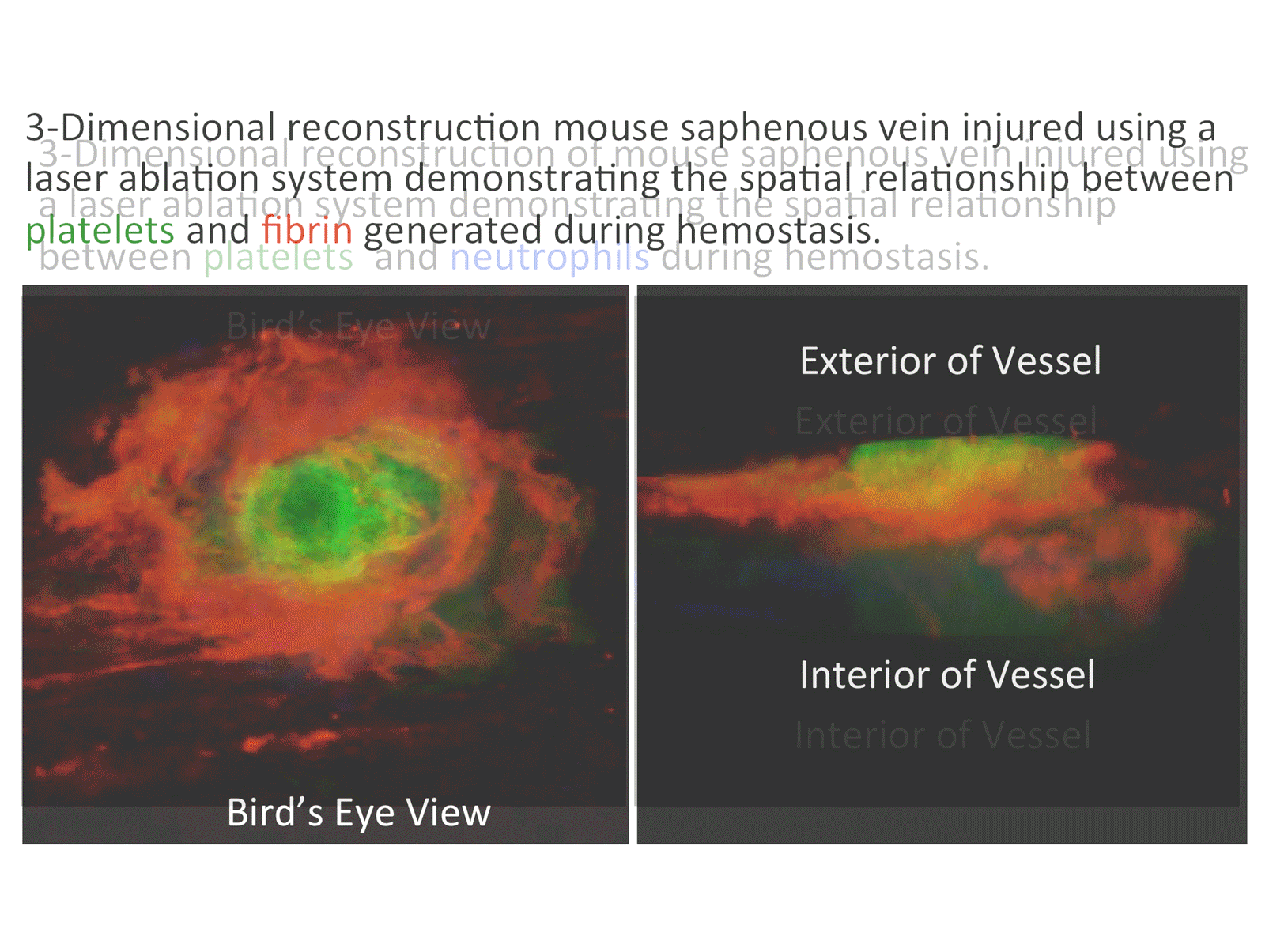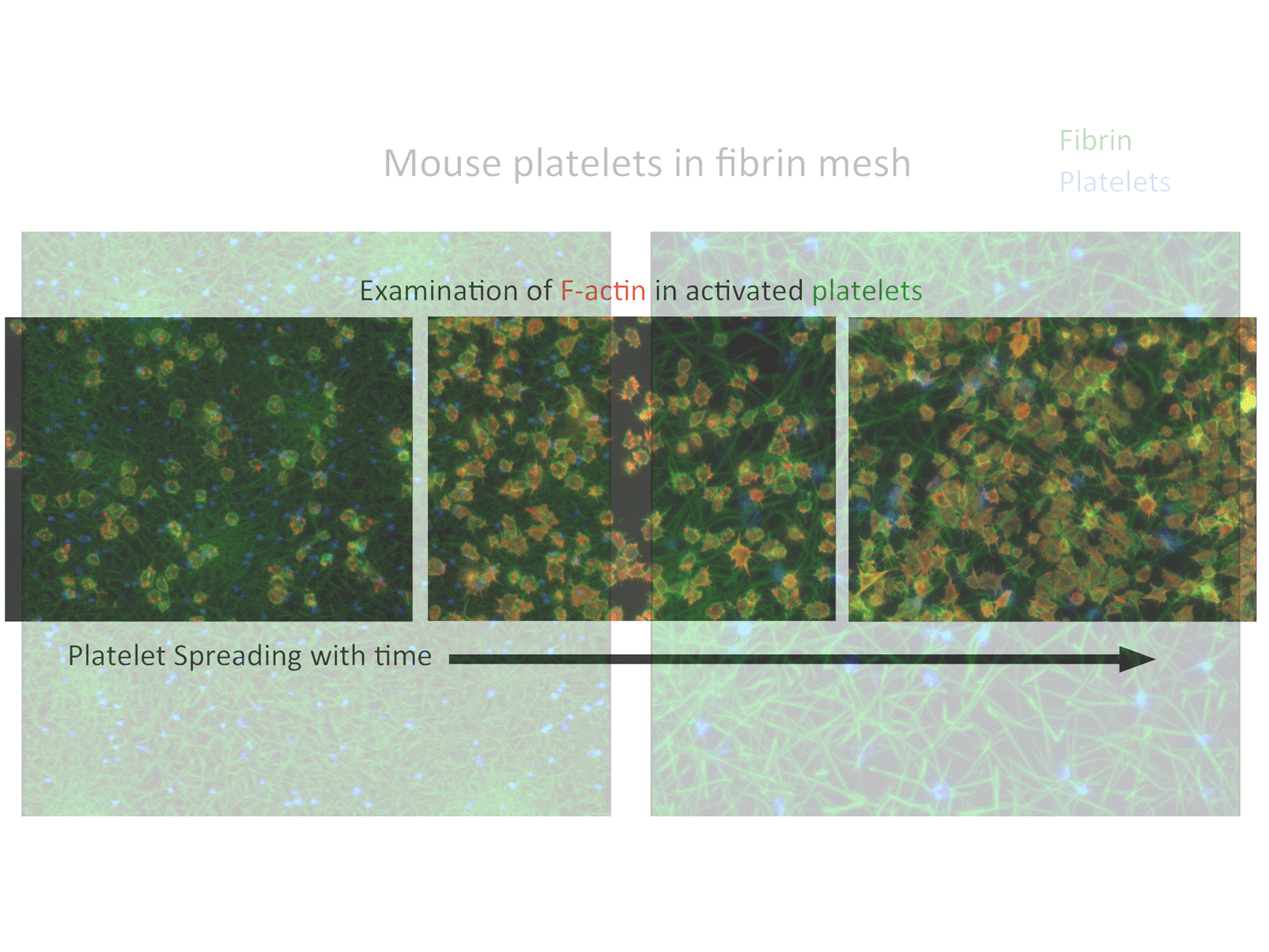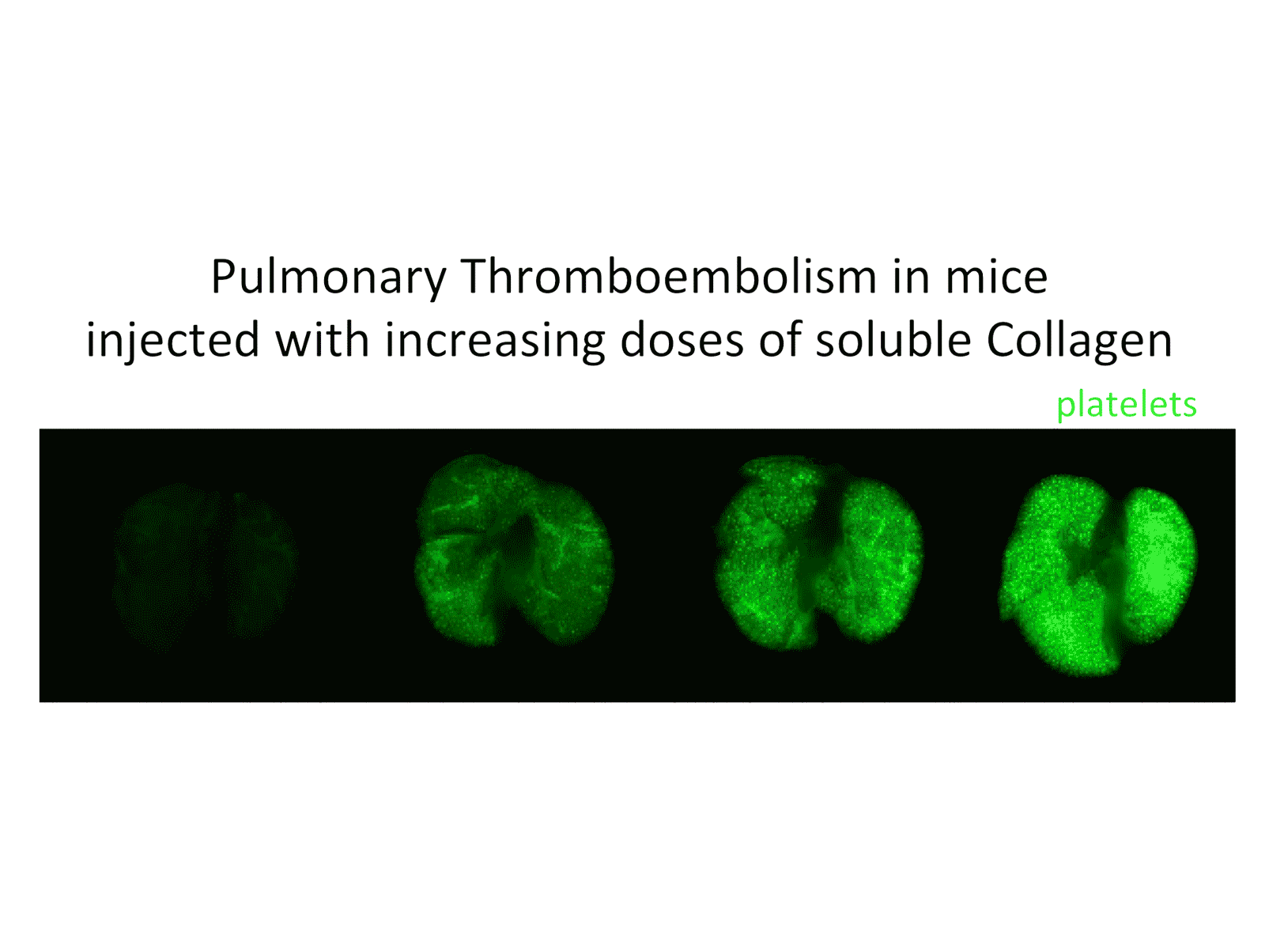
• Novel intravital imaging platforms to visualize and evaluate MK function in the bone marrow niche and the hemostatic function of mouse and human platelets at sites of vascular injury.
Central to our success are unique tools and a large network of national and international collaborators, who provide invaluable tools, samples, know-how, and feedback for our work.
Our long-term goal is to build upon these studies and devise new strategies to diagnose and treat people at risk for bleeding or thrombosis. Our over-arching hypotheses are that proper function of both MKs and platelets is strongly dependent on tight spatio-temporal control of small GTPase activity, and that disruptions in this important regulatory network lead to clinical pathologies associated with impaired platelet number and function.
Ongoing projects
(1) Biochemical/biophysical and cell biological studies to establish small GTPase activity map in MKs and platelets
Consistent with our main hypothesis that proper function of both MKs and platelets is strongly dependent on tight spatio-temporal control of small GTPase activity, we are investigating how these signaling pathways are regulated.
Important questions we are asking include:
• Are there different pools of Rap1 protein that regulate specific cellular responses in MKs and platelets?
• What are critical Rap-GEFs and -GAPs, and how is their activity regulated?
• Is there cross-talk between small GTPases, and how is this communication regulated in space and time?
• Can we manipulate small GTPase signaling to improve MK and platelet function?
(2) Preclinical and clinical work aimed at improving antiplatelet and platelet transfusion therapy
Our long-term goal is to devise new strategies to diagnose and treat people at risk for bleeding or thrombosis. Based on our recent work, we are investigating the following questions:
• Can we detect interindividual variability in Rap1 signaling in the human population?
• If yes, could a better knowledge of this variability be used to optimize antiplatelet therapy (“personalized medicine”)?
• Can we identify novel targets to more safely impair platelet function, and can such interventions be used outside of thrombosis prevention?
• Would it be beneficial to combine platelet transfusion with other hemostatic agents, such as procoagulants, antifibrinolytics, or nanoparticles?
• Can we generate novel investigational tools to bridge the gap between the bench and the bedside?
• How can we make platelet transfusion therapy more effective, i.e. should there be a more individualized approach?
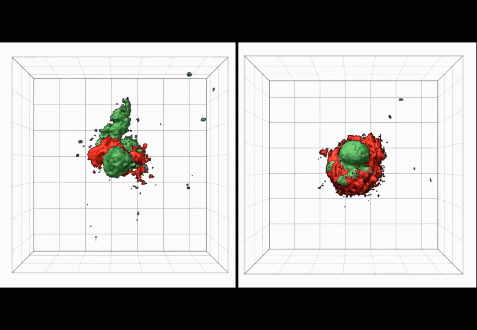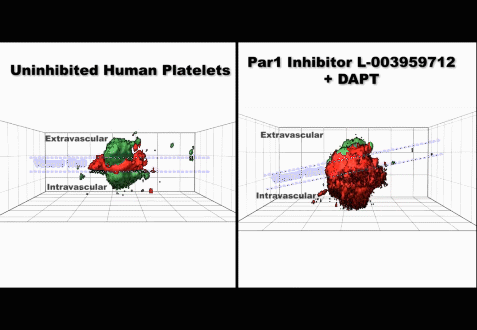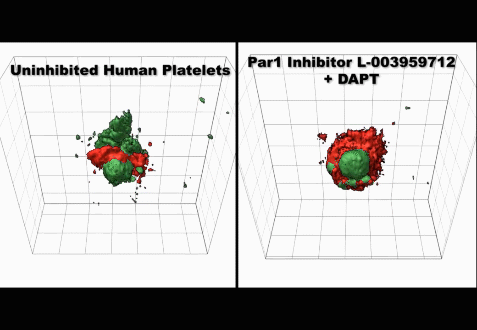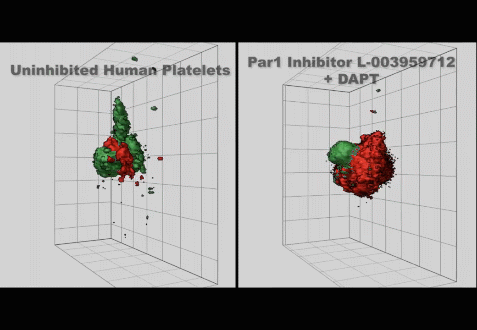

(3) Studies on the mechanisms regulating the production and clearance of platelets
Every day ~100 billion platelets are produced and destroyed by the body. Defects in either process can lead to thrombocytopenia (low platelet count) or thrombocytosis (high platelet count). While thrombocytosis is a risk factor for various clinical complications, such as venous thrombosis, atherosclerosis, and cancer, thrombocytopenia is the most common cause of bleeding complications. The fight against cancer with chemo- and radiation therapy is often accompanied by severe thrombocytopenia and bleeding. Our recent studies highlight the importance of small GTPase signaling for platelet production and survival in circulation. We have several projects that build upon these findings:
• Utilizing our unique mouse models, we are establishing Rap1 signaling controls MK function and how dysregulated integrin signaling leads to platelet clearance.
• In collaboration with the Hahn lab (department of Pharmacology) we have started to establish a spatio-temporal signature of small GTPase activity in the developing megakaryocyte. The long-term goal of this work is to improve both the yield and quality of platelets produced from stem cells in culture, an important area of research in Transfusion Medicine.
• In collaboration with the Eto lab (Kyoto University, Japan) we utilize a unique immortalized cell line to perform structure-function studies on our proteins of interest.
• With our clinical colleagues we will determine whether an imbalance in Rap1 signaling contributes to some of the inherited and/or acquired thrombocytopenias in patients.

(4) MKs/platelets in development, inflammation, atherosclerosis, and viral infection
Platelets are best known for their role in thrombosis and hemostasis. However, there is convincing evidence that platelets are also critical players in various other patho-physiological conditions, including vascular integrity in development and at sites of inflammation, plaque development in atherosclerotic blood vessels, viral infection and tumor metastasis. Excessive thrombosis, likely driven by increased platelet reactivity, is a hallmark of the Covid-19 pathology. Several of our projects investigate how platelets contribute to these processes and pathologies.